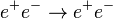Don't worry if we have covered this and you already know it. If it is too difficult, just study the bits you can understand, and come back to it later. Go to page 61
The electron (symbol: e−
) is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle.[2]
Although this may be true as far as we know, if an electron emits a photon, it could be composed of photons.
An electron has a mass that is approximately 1/1836 that of the proton.[8]
Quantum electrodynamics
Quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electrodynamics giving a complete account of matter and light interaction. One of the founding fathers of QED, Richard Feynman, has called it "the jewel of physics" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron, and the Lamb shift of the energy levels of hydrogen.[1]
In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum.
Scattering
In quantum electrodynamics, Bhabha scattering is the electron-positron scattering process:
There are two leading-order Feynman diagrams contributing to this interaction: an annihilation process and a scattering process. The Bhabha scattering rate is used as a luminosity monitor in electron-positron colliders.
Bhabha scattering is named after Indian physicist Homi J. Bhabha.
Dirac equation
In physics, more specifically relativistic quantum mechanics, the Dirac equation is a wave equation, formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and was the first theory fully to account for relativity in the context of quantum mechanics. It accounted for the fine details of the hydrogen spectrum in a completely rigorous way. The equation also implied the existence of a new form of matter, antimatter, hitherto unsuspected and unobserved, and actually predated its experimental discovery. It also provided a theoretical justification for the introduction of several-component wave functions in Pauli's phenomenological theory of spin. Although Dirac did not at first fully appreciate what his own equation was telling him, his resolute faith in the logic of mathematics as a means to physical reasoning, his explanation of spin as a consequence of the union of quantum mechanics and relativity, and the eventual discovery of the positron, represents one of the great triumphs of theoretical physics, fully on a par with the work of Newton, Maxwell, and Einstein before him.
Sine wave
The sine wave or sinusoid is a mathematical function that describes a smooth repetitive oscillation. It occurs often in pure mathematics, as well as physics, signal processing, electrical engineering and many other fields. Its most basic form as a function of time (t) is:
where:
- A, the amplitude, is the peak deviation of the function from its center position.
- ω, the angular frequency, specifies how many oscillations occur in a unit time interval, in radians per second
- ɸ, the phase, specifies where in its cycle the oscillation begins at t = 0.
- When the phase is non-zero, the entire waveform appears to be shifted in time by the amount φ/ω seconds. A negative value represents a delay, and a positive value represents an advance.
The sine wave is important in physics because it retains its waveshape when added to another sine wave of the same frequency and arbitrary phase. It is the only periodic waveform that has this property. This property leads to its importance in Fourier analysis and makes it acoustically unique.
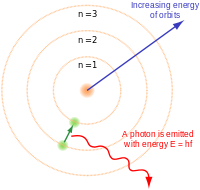
The Rutherford–Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transition depicted here produces the first line of the Balmer series, and for hydrogen (Z = 1) it results in a photon of wavelength 656 nm (red light
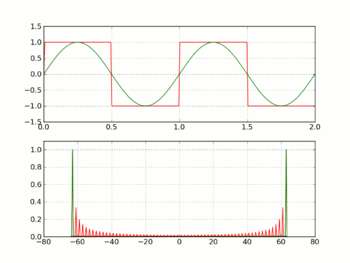
Square wave with sine wave superimposed.
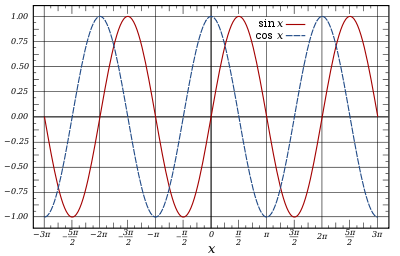
The graphs of the sine and cosine functions are sinusoids of different phases.
Synchrotron radiation
The electromagnetic radiation emitted when charged particles are accelerated radially ( ) is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers. It is similar to cyclotron radiation except that is generated by the acceleration of ultrarelativistic (moving near the speed of light) charged particles through magnetic fields. This may be achieved artificially in synchrotron or storage rings,
or naturally by fast electrons moving through magnetic fields in space.
The radiation produced in this way has a characteristic polarization and can range over the entire electromagnetic spectrum.
) is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers. It is similar to cyclotron radiation except that is generated by the acceleration of ultrarelativistic (moving near the speed of light) charged particles through magnetic fields. This may be achieved artificially in synchrotron or storage rings,
or naturally by fast electrons moving through magnetic fields in space.
The radiation produced in this way has a characteristic polarization and can range over the entire electromagnetic spectrum.
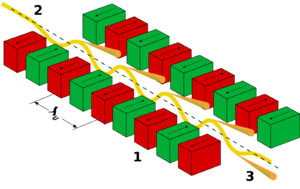
An undulator is an insertion device from high-energy physics and usually part of a larger installation, a synchrotron storage ring. It consists of a periodic structure of dipole magnets (see dipole magnet). The static magnetic field is alternating along the length of the undulator with a wavelength λu. Electrons traversing the periodic magnet structure are forced to undergo oscillations and thus to radiate energy. The radiation produced in an undulator is very intense and concentrated in narrow energy bands in the spectrum. It is also collimated on the orbit plane of the electrons. This radiation is guided through beamlines for experiments in various scientific areas.
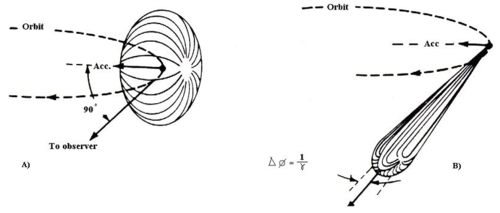
When the electron velocity approaches the speed of light, the emission pattern is sharply collimated forward. more on page 61
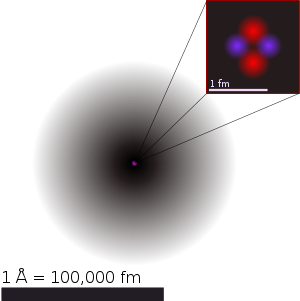
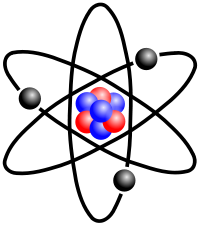
There are differing ideas about the structure of the atom. The simplist way to make an atomic bomb (fission explosion or uncontrolled nuclear chain reaction), is to use neutrons to split atoms. This is done by using radio waves to boost the (hydrogen or) helium atom (nucleus) to release neutrons. more on this page...