Just as Ernest Rutherford is credited with formulating the Rutherford model of the atom, my insight has led me to form a Baker model of the atom, based on new information about the structure of the proton and the neutron, which was understood by me late in 1983 and will be explained in thse notes.
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The proton–neutron model of nucleus was proposed by Dmitry Ivanenko in 1932.[citation needed] Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the orbiting electrons.
The diameter of the nucleus is in the range of 1.75 fm (femtometre) (1.75×10−15 m) for hydrogen (the diameter of a single proton)[1] to about 15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electronic cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).
The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.

A schematic presentation of the plum pudding model of the atom. In Thomson's mathematical model the "corpuscles" (or modern electrons) were arranged non-randomly, in rotating rings.
The plum pudding model of the atom by J. J. Thomson, who discovered the electron in 1897, was proposed in 1904 before the discovery of the atomic nucleus in order to add the electron to the atomic model. In this model, the atom is composed of electrons (which Thomson still called "corpuscles", though G. J. Stoney had proposed that atoms of electricity be called electrons in 1894[1]) surrounded by a soup of positive charge to balance the electrons' negative charges, like negatively-charged "plums" surrounded by positively-charged "pudding". The electrons (as we know them today) were thought to be positioned throughout the atom, but with many structures possible for positioning multiple electrons, particularly rotating rings of electrons (see below). Instead of a soup, the atom was also sometimes said to have had a "cloud" of positive charge.
In physics, Gauss's law, also known as Gauss's flux theorem, is a law relating the distribution of electric charge to the resulting electric field. Gauss's law states that:
The law was formulated by Carl Friedrich Gauss in 1835, but was not published until 1867.[2] It is one of the four Maxwell's equations which form the basis of classical electrodynamics, the other three being Gauss's law for magnetism, Faraday's law of induction, and Ampère's law with Maxwell's correction. Gauss's law can be used to derive Coulomb's law,[3] and vice versa.

Rutherford remains the only science Nobel Prize winner to have performed his most famous work after receiving the prize.[11] Along with Hans Geiger and Ernest Marsden in 1909 he carried out the Geiger–Marsden experiment, which demonstrated the nuclear nature of atoms. Rutherford was inspired to ask Geiger and Marsden in this experiment to look for alpha particles with very high deflection angles, of a type not expected from any theory of matter at that time. Such deflections, though rare, were found, and proved to be a smooth but high-order function of the deflection angle. It was Rutherford's interpretation of this data that led him to formulate the Rutherford model of the atom in 1911 — that a very small positively charged nucleus was orbited by electrons.
Before leaving Manchester in 1919 to take over the Cavendish laboratory in Cambridge, Rutherford became in 1917 the first person to deliberately transmute one element into another, when he converted nitrogen into oxygen through the nuclear reaction 14N + α → 17O + proton. In the products of this reaction he recognized the particle radiation from earlier experiments in which he had bombarded hydrogen gas with alpha particles, and thus as hydrogen nuclei. This result showed that hydrogen nuclei were a part of nitrogen nuclei (and by inference, probably other nuclei as well). Such a construction had been suspected for many years on the basis of atomic weights which were whole numbers of that of hydrogen; see Prout's hypothesis. Now Rutherford decided that a hydrogen nucleus was a fundamental building block and a particle, which he named the proton.
In 1921, while working with Niels Bohr (who postulated that electrons moved in specific orbits), Rutherford theorised about the existence of neutrons, which could somehow compensate for the repelling effect of the positive charges of protons by causing an attractive nuclear force and thus keeping the nuclei from breaking apart. Rutherford's theory of neutrons was proved in 1932 by his associate James Chadwick, who in 1935 was awarded the Nobel Prize in Physics for this discovery.
The Rutherford model or planetary model is a model of the atom devised by Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested on Rutherford's 1911 analysis that the so-called "plum pudding model" of J. J. Thomson of the atom was incorrect. Rutherford's new model[1] for the atom, based on the experimental results, had the new features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom and containing the bulk of the atomic mass (the nucleus of the atom).
Rutherford's model did not make any new headway in explaining the electron-structure of the atom; in this regard Rutherford merely mentioned earlier atomic models in which a number of tiny electrons circled the nucleus like planets around the sun, or a ring around a planet (such as Saturn). However, by implication, Rutherford's concentration of most of the atom's mass into a very small core made a planetary model an even more likely metaphor than before, as such a core would contain most of the atom's mass, in an analogous way to the Sun containing most of the solar system's mass. Rutherford's model was later corrected by Niels Bohr.

Spectral lines of neon in the visible region

Spectrum of neon with ultraviolet (at left) and infrared (at right) lines shown in white

In this experiment, Ernest Rutherford shot alpha particles at a thin golden foil. Based on the unexpectedly large deflection of some alpha particles, Rutherford deduced the existence of a small but heavy nucleus at the center of the atom.

Atomic model Rutherford: electrons (green) and nucleus (red).
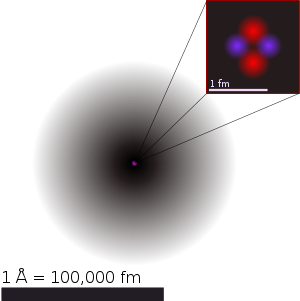
A figurative depiction of the helium-4 atom with the electron cloud in shades of gray. In the nucleus, the two protons and two neutrons are depicted in red and blue. This depiction shows the particles as separate, whereas in an actual helium atom, the protons are superimposed in space and most likely found at the very center of the nucleus, and the same is true of the two neutrons. Thus, all four particles are most likely found in exactly the same space, at the central point. Classical images of separate particles fail to model known charge distributions in very small nuclei. A more accurate image is that the spacial distribution of nucleons in helium's nucleus, although on a far smaller scale, is much closer to the helium electron cloud shown here, than to the fanciful nucleus image
Modern nuclear theory
The nucleus of an atom consists of protons and neutrons (two types of baryons) bound by the nuclear force (also known as the residual strong force). These baryons are further composed of subatomic fundamental particles known as quarks bound by the strong interaction. Which chemical element an atom represents is determined by the number of protons in the nucleus. Each proton carries a single positive charge, and the total electrical charge of the nucleus is spread fairly uniformly throughout its body, with a fall-off at the edge.

Advertise on this page: txt to (New Zealand) 027-21-567-42 £10 -12 months
Advertising is a form of communication used to encourage or persuade an audience (viewers, readers or listeners) to continue or take some new action. Most commonly, the desired result is to drive consumer behavior with respect to a commercial offering, although political and ideological advertising is also common. The purpose of advertising may also be to reassure employees or shareholders that a company is viable or successful. Advertising messages are usually paid for by sponsors and viewed via various traditional media; including mass media such as newspaper, magazines, television commercial, radio advertisement, outdoor advertising or direct mail; or new media such as websites and text messages.