Ernest Rutherford is considered by many to be the father of modern nuclear science since he came up with a new and more accurate model of the atom, which correctly described the nucleus. Up until the time of his discovery the model of the atom was described as a 'Plum pudding' in which unknows elements were distributed randomly, like fruit in a Christmas pudding.
The first evidence for isotopes of a stable element. In the bottom right corner of J. J. Thomson's photographic plate are the separate impact marks for the two isotopes neon-20 and neon-22.
When an atom or element gains a neutron, where does it come from? The obvious answer is that it must either be created inside the nucleus, or come from outside the atom, but could it come from the nucleus itself, and if so how and where? We must keep our minds open to all possibilities until we have a complete understanding of the process and can demonstrate it scientifically.

Neon is actually abundant on a universal scale; it is the fifth most abundant chemical element in the universe by mass, after hydrogen, helium, oxygen, and carbon (see chemical element). Its relative rarity on Earth, like that of helium, is due to its relative lightness, high vapor pressure at very low temperatures, and chemical inertness, all properties which tend to keep it from being trapped in the condensing gas and dust clouds which resulted in the formation of smaller and warmer solid planets like Earth.
Neon is monatomic, making it lighter than the molecules of diatomic nitrogen and oxygen which form the bulk of Earth's atmosphere; a balloon filled with neon will rise in air, albeit more slowly than a helium balloon.[21]


Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS[1] (30 August 1871 – 19 October 1937) was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics.[2] In early work he discovered the concept of radioactive half-life, proved that radioactivity involved the transmutation of one chemical element to another, and also differentiated and named alpha and beta radiation, proving that the former was essentially helium ions. This work was done at McGill University in Canada. It is the basis for the Nobel Prize in Chemistry he was awarded in 1908 "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances".[3]
Neon played a role in the basic understanding of the nature of atoms in 1913, when J. J. Thomson, as part of his exploration into the composition of canal rays, channeled streams of neon ions through a magnetic and an electric field and measured their deflection by placing a photographic plate in their path. Thomson observed two separate patches of light on the photographic plate (see image), which suggested two different parabolas of deflection. Thomson eventually concluded that some of the atoms in the neon gas were of higher mass than the rest. Though not understood at the time by Thomson, this was the first discovery of isotopes of stable atoms. It was made by using a crude version of an instrument we now term as a mass spectrometer.
Stable forms of neon are produced in stars. It is created in fusing helium and oxygen in the alpha process, which requires temperatures above 100 megakelvins and masses greater than 3 solar masses.

Neon gas in a discharge tube, so-called neon light.

Spectral lines of neon in the visible region
In addition, isotopic analysis of exposed terrestrial rocks has demonstrated the cosmogenic (cosmic ray) production of 21Ne. This isotope is generated by spallation reactions on magnesium, sodium, silicon, and aluminium. By analyzing all three isotopes, the cosmogenic component can be resolved from magmatic neon and nucleogenic neon. This suggests that neon will be a useful tool in determining cosmic exposure ages of surface rocks and meteorites.[13]
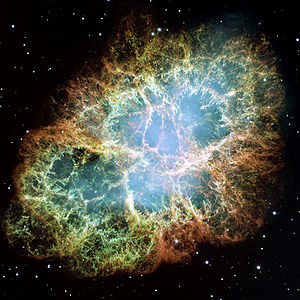

The Crab Nebula, the shattered remnants of a star which exploded as a supernova, the light of which reached Earth in 1054 AD

The onion-like layers of a massive, evolved star just before core collapse. (Not to scale.)
Neon (Greek νέον (neon) meaning "new one") was discovered in 1898 by the British chemists Sir William Ramsay (1852–1916) and Morris W. Travers (1872–1961) in London.[7] Neon was discovered when Ramsay chilled a sample of the atmosphere until it became a liquid, then warmed the liquid and captured the gases as they boiled off. The gases that boiled off, in addition to nitrogen, oxygen, and argon, were krypton, xenon, and neon.[8] The characteristic, brilliant red color that is emitted by gaseous neon when excited electrically was noted immediately; Travers later wrote, "the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget."[9]
Gaining a neutron
Neon is the second lightest inert gas. Neon has three stable isotopes: 20Ne (90.48%), 21Ne (0.27%) and 22Ne (9.25%). 21Ne and 22Ne are partly primordial and partly nucleogenic (i.e., made by nuclear reactions of other nuclides with neutrons or other particles in the environment) and their variations in natural abundance are well understood. In contrast, 20Ne (the chief primordial isotope made in stellar nucleosynthesis) is not known to be nucleogenic or radiogenic (save for cluster decay production, which is thought to produce only a small amount). The causes of the variation of 20Ne in the Earth have thus been hotly debated.[11]
The principal nuclear reactions which generate nucleogenic neon isotopes start from 24Mg and 25Mg, which produce 21Ne and 22Ne, respectively, after neutron capture and immediate emission of an alpha particle. The neutrons that produce the reactions are mostly produced by secondary spallation reactions from alpha particles, in turn derived from uranium-series decay chains. The net result yields a trend towards lower 20Ne/22Ne and higher 21Ne/22Ne ratios observed in uranium-rich rocks such as granites.[12] Neon-21 may also be produced in a nucleogenic reaction, when 20Ne absorbs a neutron from various natural terrestrial neutron sources.
Similar to xenon, neon content observed in samples of volcanic gases is enriched in 20Ne, as well as nucleogenic 21Ne, relative to 22Ne content. The neon isotopic content of these mantle-derived samples represents a non-atmospheric source of neon. The 20Ne-enriched components are attributed to exotic primordial rare gas components in the Earth, possibly representing solar neon. Elevated 20Ne abundances are found in diamonds, further suggesting a solar neon reservoir in the Earth.[14]

Advertise on this page: txt to (New Zealand) 027-21-567-42 £10 -12 months
Advertising is a form of communication used to encourage or persuade an audience (viewers, readers or listeners) to continue or take some new action. Most commonly, the desired result is to drive consumer behavior with respect to a commercial offering, although political and ideological advertising is also common. The purpose of advertising may also be to reassure employees or shareholders that a company is viable or successful. Advertising messages are usually paid for by sponsors and viewed via various traditional media; including mass media such as newspaper, magazines, television commercial, radio advertisement, outdoor advertising or direct mail; or new media such as websites and text messages.