Why choose five different particles to build the model for the proton and the neutron? Call it a hunch, or the result of careful investigation. Soon we will get on to prisms, which break up light, and describe how they do this, and explain another conclusion, that photons are not the smallest, or fastest matter in the universe. They simply, logically, cannot be. More later.
If one of the sub-atomic structures which constututes the proton immitates the structure of the Hydrogen atom (we can call it bHydrogen), then we can call the others bHelium, bLithium, bBeryllium and bBoron. The next level down (inside) we can call cHydrogen, cHelium, and so on. The next level is dHydrogen, eHydrogen and fHydrogen, but we don't know whether the nucleus is simple like the Hydrogen atom itself, or complex like the proton, which has five (or three) sub-atomic structures within it.
Do we have enough information to draw this conclusion, seeing that all the scientists are going in a different direction of enquiry?
Clearly experiments must be designed to draw energy from teh nucleus of this new model, but the mathematics does support my model. We can look at teh same set of facts and draw different conclusions, simply because the scale is so small we have no way of 'seeing' what is there.
Cosmic rays
Cosmic rays are energetic charged subatomic particles, originating from outer space. They may produce secondary particles that penetrate the Earth's atmosphere and surface. The term ray is historical as cosmic rays were thought to be electromagnetic radiation. Most primary cosmic rays (those that enter the atmosphere from deep space) are composed of familiar stable subatomic particles that normally occur on Earth, such as protons, atomic nuclei, or electrons. However, a very small fraction are stable particles of antimatter, such as positrons or antiprotons, and the precise nature of this remaining fraction is an area of active research.
About 89% of cosmic rays are simple protons or hydrogen nuclei, 10% are helium nuclei or alpha particles, and 1% are the nuclei of heavier elements. These nuclei constitute 99% of the cosmic rays. Solitary electrons (much like beta particles, although their ultimate source is unknown) constitute much of the remaining 1%.
The obscure mechanism of cosmic ray production at galactic distances is partly a result of the fact that (unlike other radiations) magnetic fields in our galaxy and other galaxies bend cosmic ray direction severely, so that they arrive nearly randomly from all directions, hiding any clue of the direction of their initial sources. Cosmic rays can have energies of over 1020 eV, far higher than the 1012 to 1013 eV that Terrestrial particle accelerators can produce. There has been interest in investigating cosmic rays of even greater energies.[1]
Cosmic rays are enriched in lithium, beryllium, and boron with regard to the relative abundance of these elements in the universe compared to hydrogen and helium, and thus are thought to have a primary role in the synthesis of these three elements through the process of "cosmic ray nucleosynthesis". They also produce some so-called cosmogenic stable isotopes and radioisotopes on Earth, such as carbon-14.[2] In the history of particle physics, cosmic rays were the source of the discovery of the positron, muon, and pi meson.
We hnow that Plancks constant is:
Since the frequency ν, wavelength λ, and speed of light c are related by λν = c, the Planck relation can also be expressed as
The wavelength λ of a sinusoidal waveform traveling at constant speed v is given by:
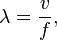
- where v is called the phase speed (magnitude of the phase velocity) of the wave and f is the wave's frequency.
The first six elements of the periodic table.
Wavelength
Another property of an electromagnetic wave is its wavelength. The wavelength is inversely proportional to the frequency, so an electromagnetic wave with a higher frequency has a shorter wavelength, and vice-versa.
The wavelength λ of a sinusoidal waveform traveling at constant speed v is given by:[8]
where v is called the phase speed (magnitude of the phase velocity) of the wave and f is the wave's frequency.
Assuming a sinusoidal wave moving at a fixed wave speed, wavelength is inversely proportional to frequency: waves with higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths.[6]
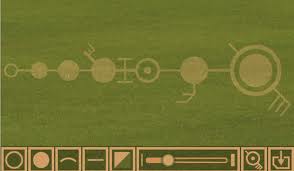
Above is an imaginary structure (it is a crop circle you can see has been made by humans, but it serves the purpose of illustration) of the (Hydrogen) atom proton, down to the (left to right) g level. Between d and e is a right angle. Between the levels is a line representing an energy pathway.
The interior is in four dimension (one down to ward the inside) but you still think of them as three dimensional, not flat.
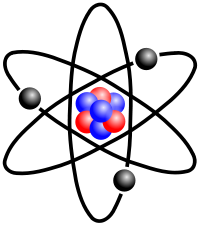
An article published in 2007 featuring a model-independent analysis concluded that the neutron has a negatively charged exterior, a positively charged middle, and a negative core.[14] In a simplified classical view, the negative "skin" of the neutron assists it to be attracted to the protons with which it interacts in the nucleus. However, the main attraction between neutrons and protons is via the nuclear force, which does not involve charge.
The energy which holds the nucleus (of the hydrogen or helium atom) together is radio (waves). The nucleus is orbiting around a central mass. To boost an element of the mass into a larger orbit, sufficient 'split' the atom causing a fission reaction, there must be sufficient quanta of energy put into the atom. If there is not enough energy in the Hydrogen nucleus, no fission reaction can be caused. If the theory is correct, even nucleus containing a (pair of) neutrons can be split.
The nuclear force
The nuclear force (or nucleon-nucleon interaction or residual strong force) is the force between two or more nucleons. It is responsible for binding of protons and neutrons into atomic nuclei. The energy released causes the masses of nuclei to be less than the total mass of the protons and neutrons which form them. The force is powerfully attractive between nucleons at distances of about 1 femtometer (fm) between their centers, but rapidly decreases to insignificance at distances beyond about 2.5 fm. At very short distances less than 0.7 fm, it becomes repulsive, and is responsible for the physical size of nuclei, since the nucleons can come no closer than the force allows.
Therefore, if we know what E (Energy) is, and what v (velocity) is, (say it is the speed of light, and we know what the wavelength is) we can work out what h is in:

- But what if we don't E is?
- λν = c, but can we be certain?
- Try a few examples your-self.
The proton of the Hydrogen atom (1 proton) is much smaller than the nucleus of the Helium atom (2 protons and 2 neutrons).
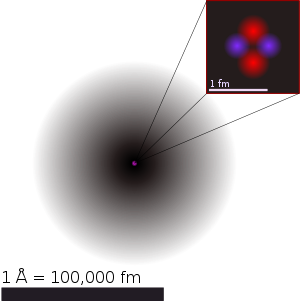
The Helium nucleus, with four (or five) particles, two protons and two neutrons. This makes 12 (quarks) according to the standard model.
Relative mass; proton to neutron
Proton
Charge radius 0.8775(51) fm[1]
Mass 1.672621777(74)×10−27 kg[1]
Neutron
Mass 1.674927351(74)×10−27 kg[3]
This won't mean much, except the are about the same size, with the neutron slightly bigger.
What does it mean? A proton holds on to an electron, the neutron is essentially the same thing but can't. It is important, because without electrons, matter has no molecules. Fortunately, all matter has protons in the nucleus. What causes a proton and a neutron to stay locked together? If we knew that (exactly) we would have the right to claim a Nobel Prize.
From Nuclear Force- on this page, we can deduce that something is revolving/orbiting, but that when it gets out of phase (reduces its orbital distance) it starts to repel instead of attract. If we stopped looking for an attracting force (gravity) we might have an answer. It should be transferred momentum (by gravity). It means looking for a group ot things, not a single solid mass.
Hydrogen
Helium

Lithium
Beryllium


Boron