This is where it gets interesting.
Here is the problem again. F=MA, P=MV.
Despite the apparent contradiction in this statement, (page x) in a scenario where velocity is not in a straight line, but is rotational, (spin) the force of F could be equal to the force P (momentum).
To make straight line vecocity into rotational velocity of an equal speed (ie, past a point of reference) there must be an increase of energy because straight line velocity is not equal to accelleration.
Can Momentum be equal to Force, if one merely applies a change of direction? Well apparently, if the force which applies a change of direction (gravity) adds no more velocity to an object, then it adds no energy to it. Is that possible in quantum mechanics? It is easy to calculate increased momentum using gravity, but there appears to be a problem because an object in a constant orbit (energy level) picks up no energy, (nor loses any). Is this important? It is from a mathematical point of view, because o the way we calculate accelleration with respect to time when the time periods are very small.
We know, because of Neptune and Pluto that energy can be transferred from one mass to another through the force of gravity, and is proportional to mass.
Atomic number
In chemistry and physics, the atomic number (also known as the proton number) is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element. In an atom of neutral charge, the atomic number is also equal to the number of electrons.
The atomic number, Z, should not be confused with the mass number, A, which is the total number of protons and neutrons in the nucleus of an atom. The number of neutrons, N, is known as the neutron number of the atom; thus, A = Z + N. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes), and the mass defect is usually very small compared to the mass, the atomic mass of an atom is roughly equal to A.
Atoms having the same atomic number Z but different neutron number N, and hence different atomic mass, are known as isotopes. Most naturally occurring elements exist as a mixture of isotopes, and the average atomic mass of this mixture determines the element's atomic weight.
The conventional symbol Z presumably comes from the German word Atomzahl (atomic number).
Atomic weight
The use of the name "atomic weight" has attracted a great deal of controversy among scientists.[5] Objectors to the name usually prefer the term "relative atomic mass" (not to be confused with atomic mass). The basic objection is that atomic weight is not a weight, that is the force exerted on an object in a gravitational field, measured in units of force such as the newton.
First six elements of the periodic table.
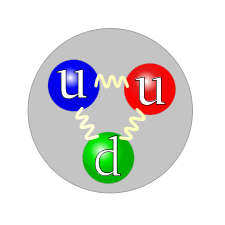
The Standard Model for protons an neutrons says there are three quarks inside the nucleus. Using only logic and P=MV, it is more realistic to suppose that there are in fact five. Whether it turns out that there are three, or five or more than five, it is most likely that it is an odd number, but not necessarily a prime number.
The good news is that there is only one type of proton, and one type of neutron, at least in teh most basic of the elements.
There is something fundamental, and simple about the Hydrogen atom which has only electron charge at the nucleus, and one electron in its energy shell.
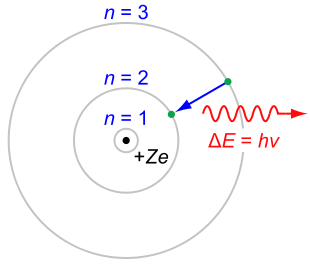
Model of Hydrogen atom (left).
Electron configuration of hydrogen atom (right).
To the left, the Helium nucleus and to the right, the same thing showing two electrons in the outside shell. The shell is region which has room for a limited number of electrons, depending on volume.
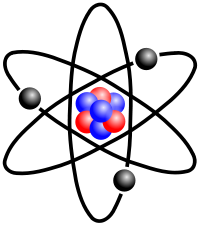
Depiction of Lithium atom, with three electrons (one in outer 'valence' shell, three protons, and four neutrons.
Lithium 6.941(2) (Atomic weight)
It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element.
The nuclei of lithium verge on instability, since the two stable lithium isotopes found in nature have among the lowest binding energies per nucleon of all stable nuclides. Because of its relative nuclear instability, lithium is less common in the solar system than 25 of the first 32 chemical elements even though the nuclei are very light in atomic weight.[1] For related reasons, lithium has important links to nuclear physics. The transmutation of lithium atoms to helium in 1932 was the first fully man-made nuclear reaction, and lithium deuteride serves as a fusion fuel in staged thermonuclear weapons.
Like the other alkali metals, lithium has a single valence electron that is easily given up to form a cation.[2] Because of this, it is a good conductor of heat and electricity as well as a highly reactive element, though the least reactive of the even-more highly reactive alkali metals. Lithium's low reactivity compared to other alkali metals is thought to be due to the proximity of its valence electron to its nucleus (the remaining two electrons in lithium's 1s orbital and are much lower in energy, and therefore they do not participate in chemical bonds).[2]
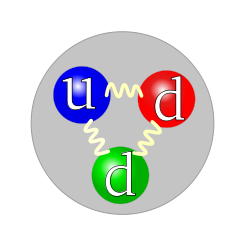
The quark structure of the neutron.

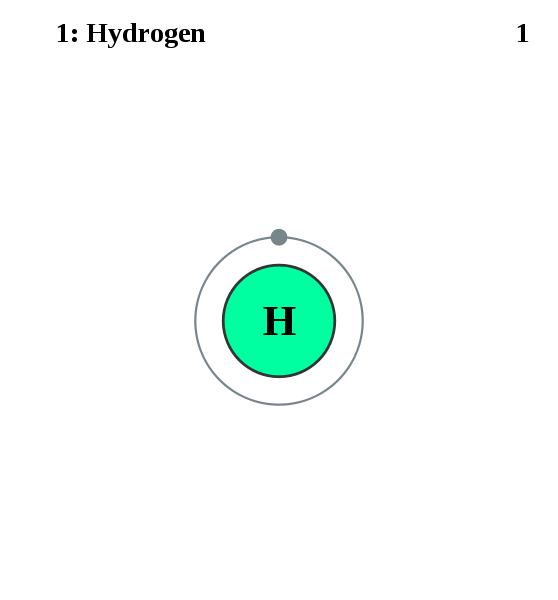
Helium atom (second) above.
Hydrogen atom, showing one electron in outside shell.
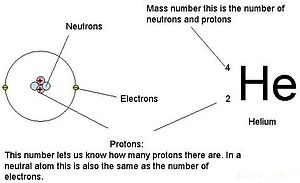
An explanation of the superscripts and subscripts seen in atomic number notation.
In what way could helium combine with another element to form a molecule?

Lithium atom showing three electrons orbiting. There is one electron in the outermost shell..
Another way to think about the Hydrogen atom (or the proton) is as a boiled egg. It has an outside shape, and an inside, the yolk (or nucleus), but think about the yolkas a bunch of smaller boiled eggs. You can spin a boiled egg on a hard surface, and it will settle on its side. Inside the atom there could be two boiled eggs one above the other, both spinning, but big end to small end, or they could be spinning in opposite directions.
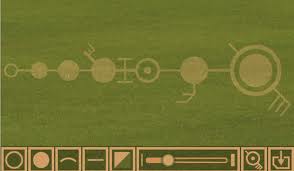
Let this diagram represent (the hollow figure at teh left) the hydrogen atom. Inside the proton, is another 'proton', smaller, represented by the second (solid) shape from the left, and inside that, another (even smaller) 'proton' or structure. It still has a simple nucleus (one positive charge) on teh outside, and a nucleus- which I will describe later.
The fourth level (down) shows something coming in (or going out) at a specific angle, and a small branch describing how the energy (or quanta) combine prior to entering the nucleus. We can number the levels, b, c, d and e.