Atoms are made of a nucleus, consisting of a proton or protons, and a neutron or neutrons, surrounded by a cloud, or shell of electrons. The exception is Hydrogen, the simplist and most abundant atom, or element, in the known universe. Hydrogen has only a proton, and no neutron.
The proton holds, by a force we do not fully understand, except that it is probably the same gravitational force which keeps the moon revolving atound the earth, and the earth and the planets revolving around the sun, one electron (force) in rotation around it.
However there is a complication that a mass of atoms although they all have a finite amount of electrons bound to their nucleii (nucleus's), can attract and discharge electrons freely, as in when a battery is charged, and discharges through a wire.
There is also the complication of magnetic force, which sometimes appears to act against gravity.
What does it look like?
An electron has a mass that is approximately 1/1836 that of the proton
Imagine mercury, ( the only metal that is liquid at standard conditions for temperature and pressure); which has a structure, like water, we know, that is composed of something smaller- in the case of water, molecules of hydrogen and oxygen, and in the case of an electron, something we haven't defined or named, but which we know is light, or photons.
This electron forms a single unit, or flows through a conductor (wire) like water flows through a pipe, except it is like a pipe full of balls, where you put one in at one end, and a different one drops out at the other end.
If it looks like a drop of silvery mercury, it is a pyramid shape, with three corners at the bottom, and one corner at the top, but it is like a balloon, because it always keeps the same mass, (size) even though it is flexible, and liquid.
It rotates around a nucleus, in the same way that the earth rotates around the sun, but it does it so rapidly that it takes up all the space around the centre of mass (proton), and if you try to add another, the other gtets knocked immediately back out of the orbit.
The only way to fit another electron to the proton of the Hydrogen atom is to add something (another proton) to the mass of the nucleus, (and why does the next element -Helium, have two neutrons as well as two protons), then the nucleus is pushed just far enough apart to let one more electron into this mini 'solar system'.
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of 1.00794 u (1.007825 u for Hydrogen-1), hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He.
In the perspective of quantum mechanics, helium is the second simplest atom to model, following the hydrogen atom. Helium is composed of two electrons in atomic orbitals surrounding a nucleus containing two protons along with some neutrons. As in Newtonian mechanics, no system consisting of more than two particles can be solved with an exact analytical mathematical approach (see 3-body problem) and helium is no exception. Thus, numerical mathematical methods are required, even to solve the system of one nucleus and two electrons. Such computational chemistry methods have been used to create a quantum mechanical picture of helium electron binding which is accurate to within < 2% of the correct value, in a few computational steps.[36] In such models it is found that each electron in helium partly screens the nucleus from the other, so that the effective nuclear charge Z which each electron sees, is about 1.69 units, not the 2 charges of a classic "bare" helium nucleus.
The nucleus of the helium-4 atom is identical with an alpha particle.
The electron (symbol: e−
) is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle.[2] An electron has a mass that is approximately 1/1836 that of the proton.[8]
So, what is gravity?
Expressed in its most simple terms it is angular momentum.
Momentum is the tendency for an object in space to continue on its trajectory.
For example, if you fire a billiard ball at another on a pool table, you can determine at what angle and velocity (speed) a mass will travel after a colision by knowing the mass and velocity of the striking ball. The formula is the fundamental one of science, F=MA (Force is equal to Mass times accelleration), and P=MV (Momentum is equal toMass times Velocity. It is the same as E= MC squared, (oe E= MC cubed).
Angular momentum is just momentum split into various components (factors).

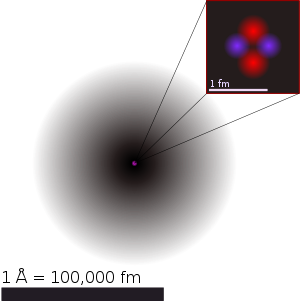
Helium nucleus
Mercury has a unique electronic configuration where electrons fill up
all the available 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d and
6s subshells. As such configuration strongly resists removal of an
electron, mercury behaves similarly to noble gas
elements, which form weak bonds and thus easily melting solids. The
stability of the 6s shell is due to the presence of a filled 4f shell.
An f shell poorly screens the nuclear charge that increases the
attractive Coulomb interaction of the 6s shell and the nucleus (see lanthanide contraction). The absence of a filled inner f shell is the reason for the somewhat higher melting temperature of cadmium and zinc, although both these metals still melt easily and, in addition, have unusually low boiling points. Metals such as gold have atoms with one less 6s electron than mercury.
A complete explanation of this fact requires a deep excursion into quantum physics.
The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion.
Personally I'd be very cautious about the following statements:
The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may either scatter off each other or be totally annihilated, producing a pair (or more) of gamma ray photons. Electrons, which belong to the first generation of the lepton particle family,[9] participate in gravitational, electromagnetic and weak interactions.[10]
Electrons, like all matter, have quantum mechanical properties of both particles and waves, so they can collide with other particles and can be diffracted like light. However, this duality is best demonstrated in experiments with electrons, due to their tiny mass. Since an electron is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[9]
The concept of an indivisible quantity of electric charge was theorized to explain the chemical properties of atoms, beginning in 1838 by British natural philosopher Richard Laming;[4] the name electron was introduced for this charge in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle in 1897 by J. J. Thomson and his team of British physicists.[6][11][12]
Classical mechanics
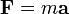
In physics, angular momentum, moment of momentum, or rotational momentum[1][2] is a conserved vector quantity that can be used to describe the overall state of a physical system. The angular momentum L of a particle with respect to some point of origin is
where r is the particle's position from the origin, p = mv is its linear momentum, and × denotes the cross product.
In physics, angular momentum is the rotational counterpart of momentum. A spinning flywheel has angular momentum.
The magnitude L of the angular momentum of a particle is
where r is the particle's distance from the origin, p = mv is the magnitude of the particle's linear momentum, and θ is the angle between the particle's position vector and its momentum vector. Angular momentum is a vector quantity and its direction is determined by applying the right-hand rule.
In the SI system of units, angular momentum is measured in kg.m2.s−1. In contrast, linear momentum is measured in units of kg.m.s−1 so the two are not compatible and cannot be added.
The angular momentum of a symmetrical body, such as a spinning flywheel, can be calculated as the product of the body's moment of inertia and its angular velocity.
To calculate the angular momentum of a rigid body or system, a point called the origin is chosen. For convenience this might be on the axis of rotation of the rigid body or the center of mass of the system. The distance from the origin to each element of the rigid body or system is determined. This is then multiplied by the transverse component of the linear momentum of each part. The angular momentum of the rigid body or system is the vector sum of all the elements.
Quantum mechanics, also known as quantum physics or quantum theory, is a theory of physics providing a mathematical description of the interaction of matter and energy. The theory was developed in 1925 by Werner Heisenberg.[1] Quantum mechanics describes the time evolution of physical systems via a mathematical structure called the wave function. The wave function encapsulates the probability that the system is to be found in a given state at a given time. Quantum mechanics also allows one to calculate the effect on the system of making measurements of properties of the system by defining the effect of those measurements on the wave function. This leads to the well-known uncertainty principle as well as the enduring debate over the role of the experimenter, epitomised in the Schrödinger's Cat thought experiment.
The macroscopic scale is the length scale on which objects or processes are of a size which is measurable and observable by the naked eye.
When applied to phenomena and abstract objects, the macroscopic scale describes existence in the world as we perceive it, often in contrast to experiences (microscopy) or theories (microphysics, statistical physics) considering objects of geometric lengths smaller than one millimeter.
A macroscopic view of a ball is just that: a ball. A microscopic view could reveal a thick round skin seemingly composed entirely of puckered cracks and fissures (as viewed through a microscope) or, further down in scale, a collection of molecules in a roughly spherical shape.
Coulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism.[1][2] Nevertheless, in 1767 Joseph Priestley of England conjectured that the force between charges varied as the inverse squared of the distance.[3][4] In 1769, Scottish physicist John Robison announced that according to his measurements, the force of repulsion between two spheres with charges of the same sign varied as x-2.06.[5] The dependence of the force between charged bodies upon both distance and charge had been discovered, but not published, in the early 1770s by Henry Cavendish of England, prior to Coulomb's works.[6]

